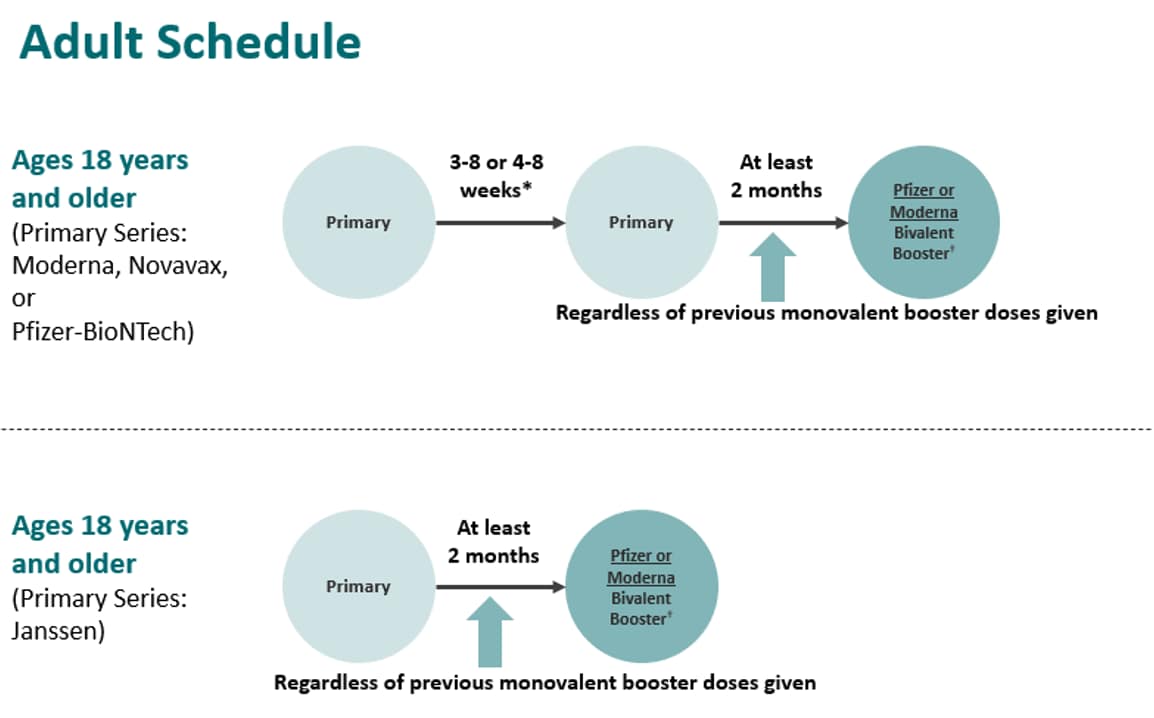
ACIP Evidence to Recommendations (EtR) for Use of Novavax COVID-19 Vaccine Booster Dose for adults ages 18 years and older under an Emergency Use Authorization | CDC
Al via martedì 1 marzo le somministrazioni ai maggiorenni del nuovo vaccino di Novavax, con prenotazione obbligatoria dal 26 febbraio — Azienda USL di Bologna

Centers for Disease Control and Prevention on LinkedIn: CDC recommends Novavax's non-mRNA booster for people ages 18 and older…

Vaccinazione covid: Novavax e quarta dose fragili / Coronavirus: tutti gli aggiornamenti / Comunicazione / Comune / Comune di Arco - Comune di Arco
Covid19, nell'Isola si parte con Novavax e la quarta dose per i soggetti fragili - Azienda Ospedaliero-Universitaria di Cagliari
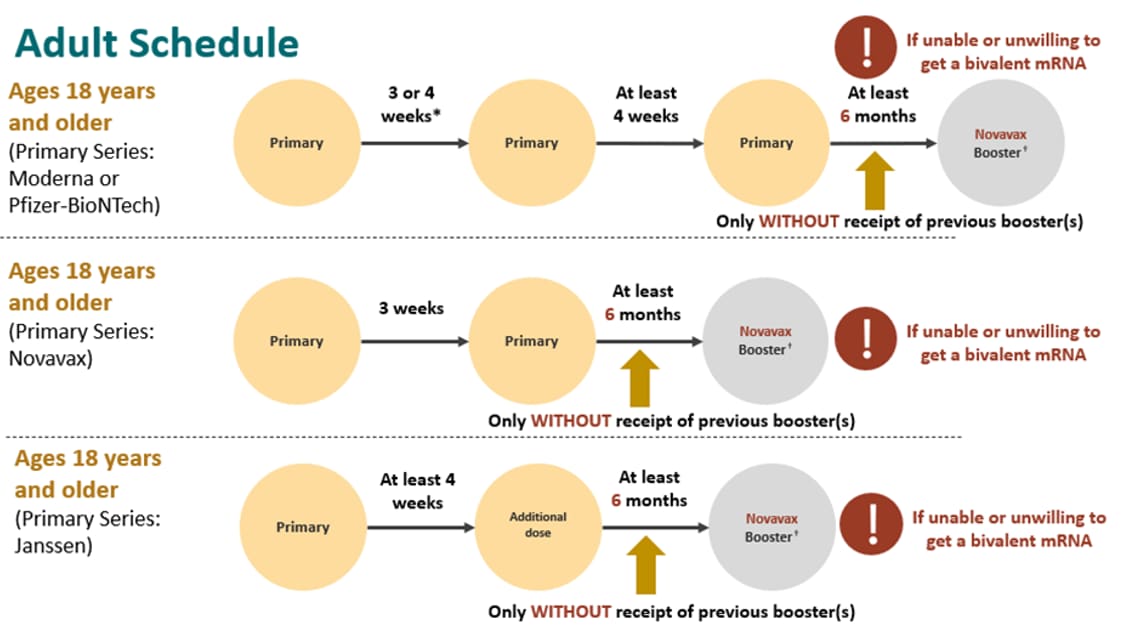

/cloudfront-us-east-1.images.arcpublishing.com/gray/COUI7Y75YBEDLGZLB356FFMMC4.jpg)