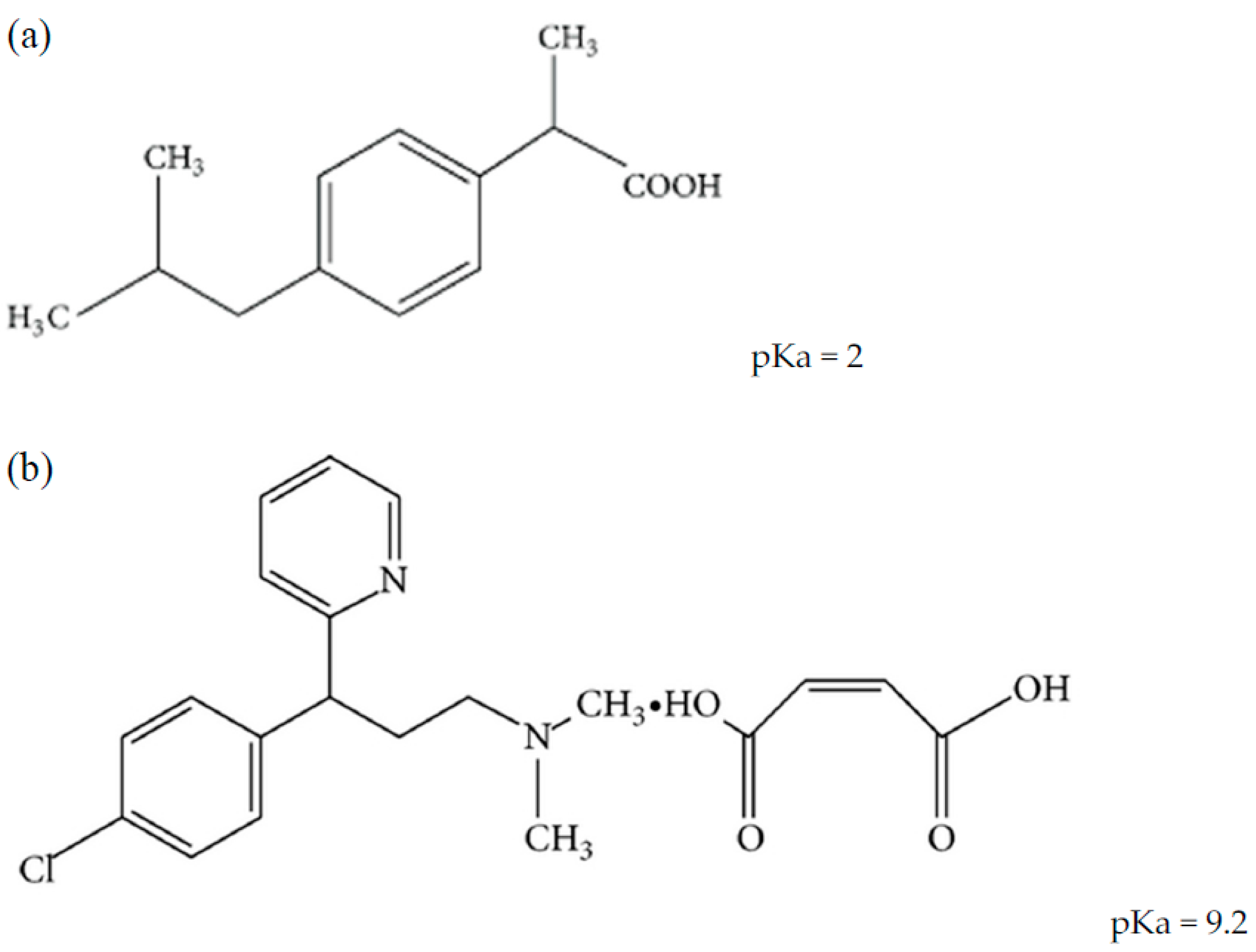
Sci. Pharm. | Free Full-Text | Development of HPLC Method for Simultaneous Determination of Ibuprofen and Chlorpheniramine Maleate

Multiple binding modes of ibuprofen in human serum albumin identified by absolute binding free energy calculations | bioRxiv

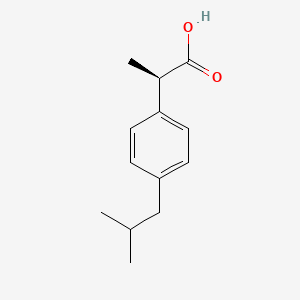
Chemical structure of ibuprofen and fenoprofen ; (*) denotes the chiral... | Download Scientific Diagram
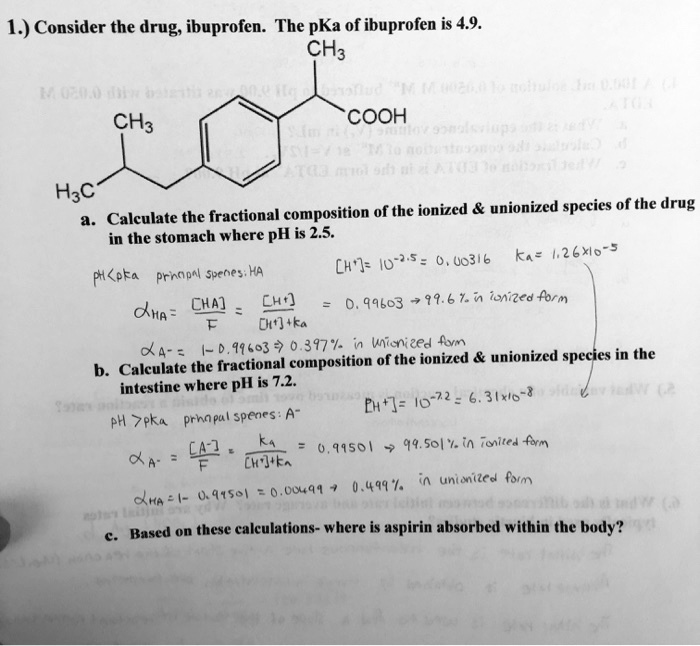
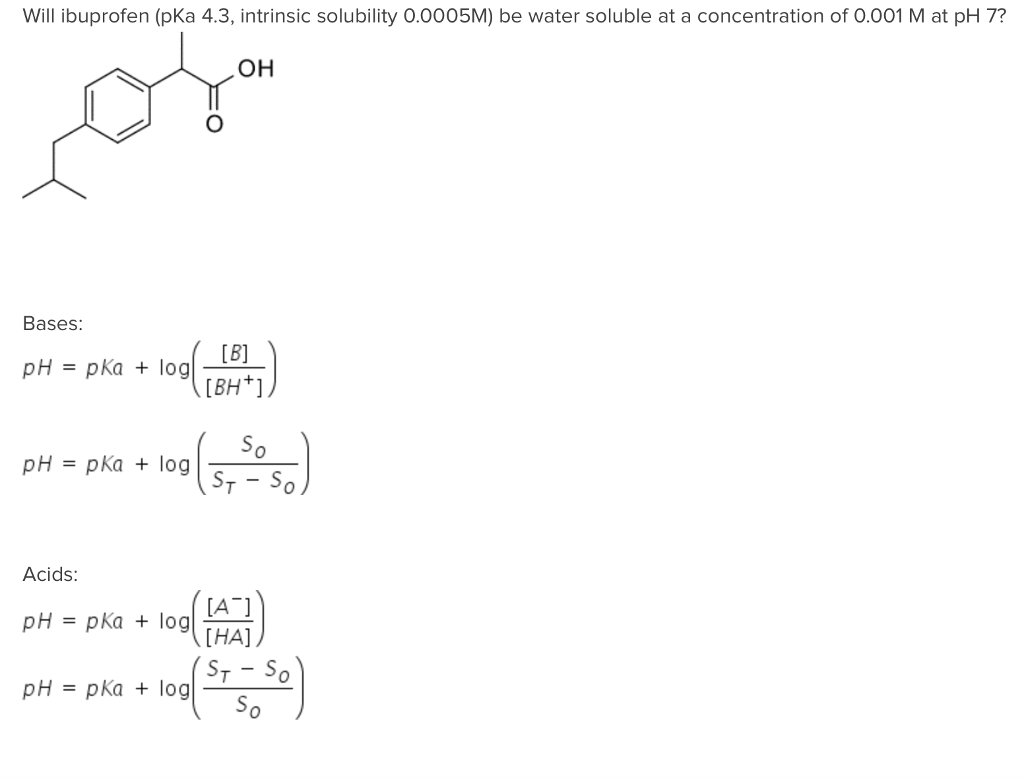
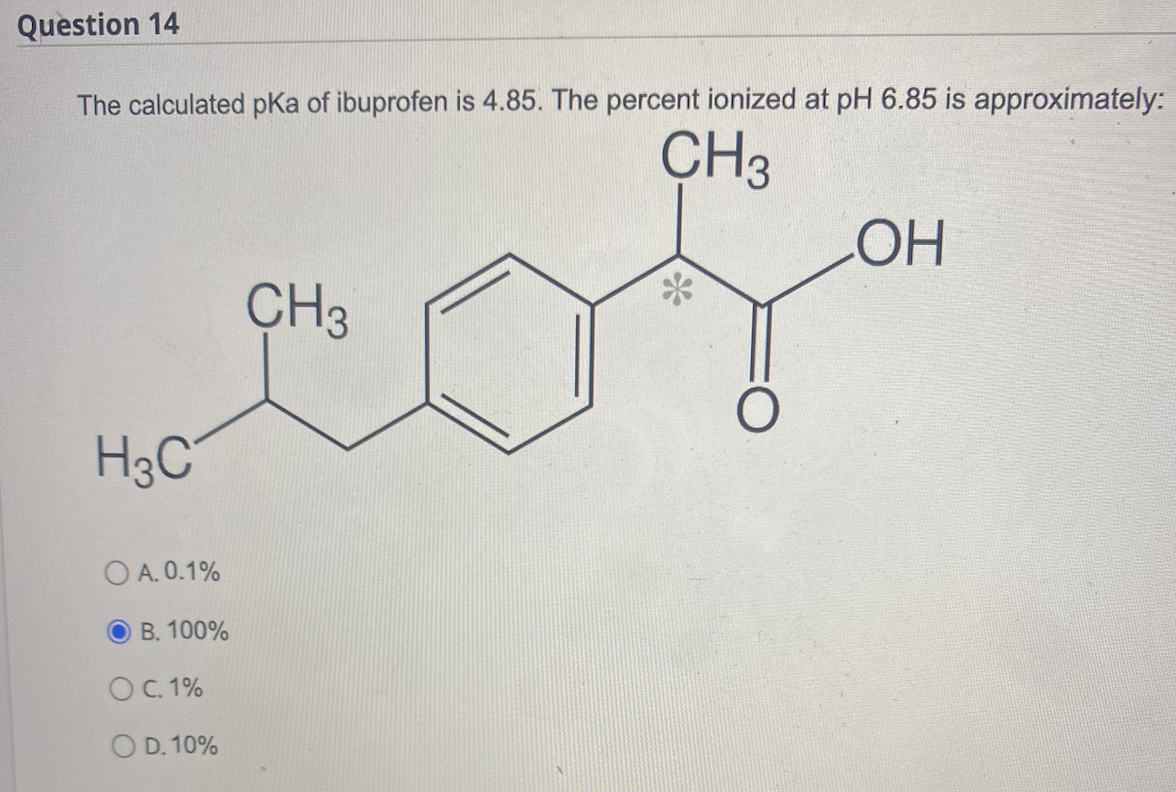
SOLVED: Consider the drug ibuprofen. The pKa of ibuprofen is 4.9. CH3COOH is the fractional composition of the ionized and unionized species of the drug. Calculate in the stomach where pH is
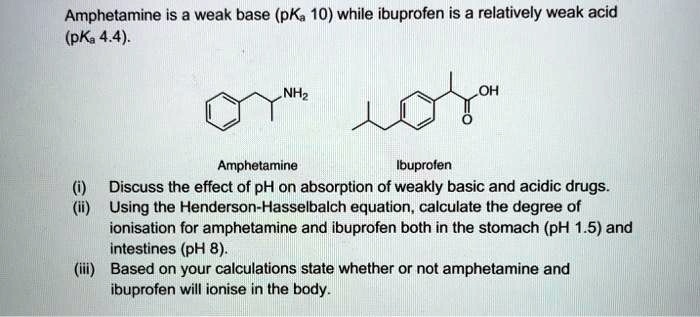
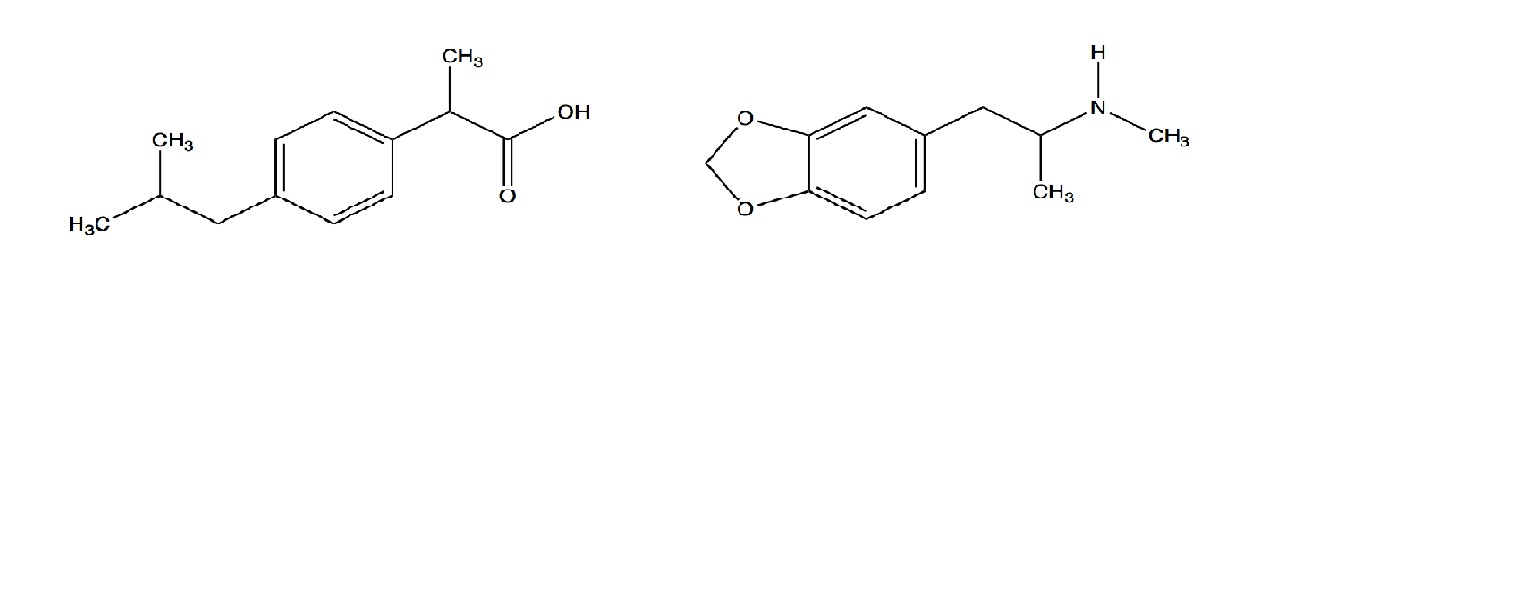
SOLVED: Amphetamine is a weak base (pKa 10) while ibuprofen is a relatively weak acid (pKa 4.4). Discuss the effect of pH on absorption of weakly basic and acidic drugs. Using the

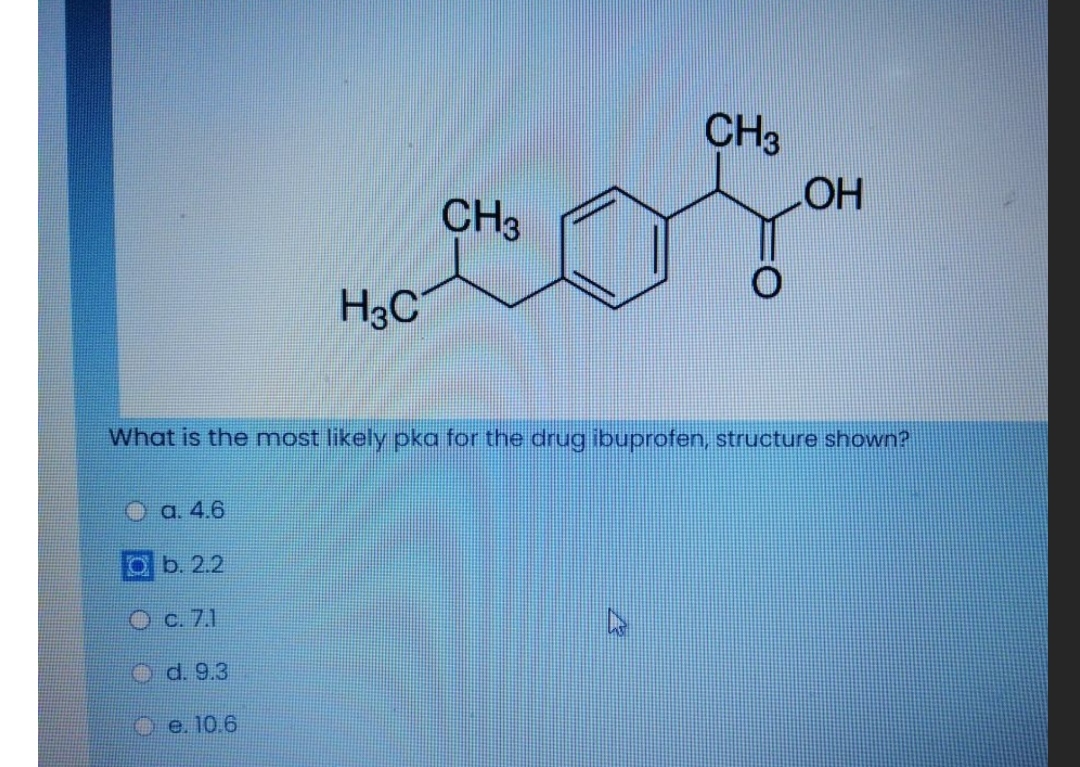
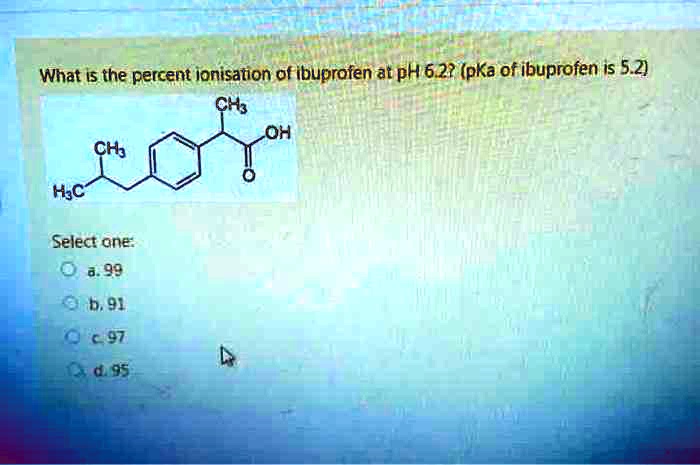
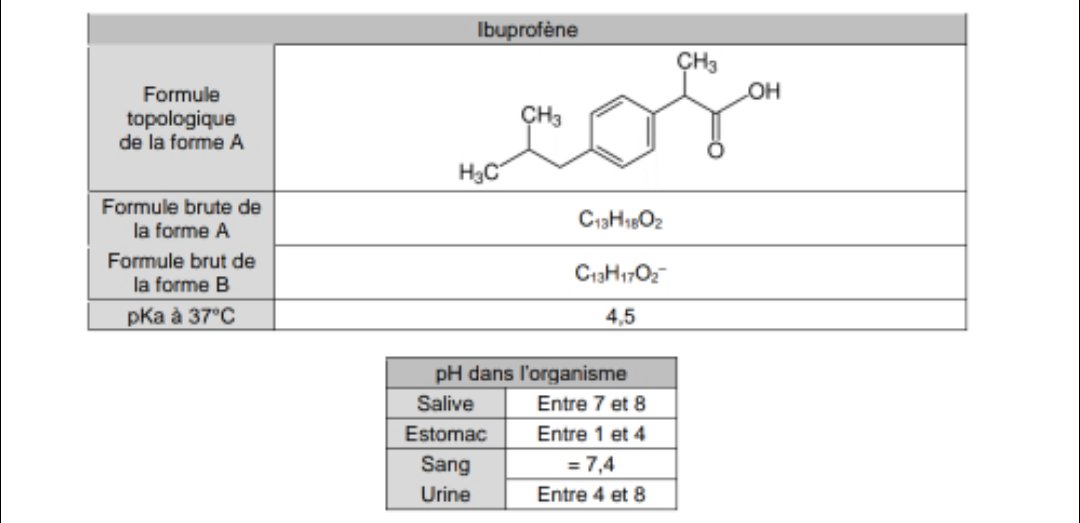
Figure1: Ibuprofen with a molecular weight of 206.3, pKa of 4.9, and... | Download Scientific Diagram

Ibuprofen: water affinity, effect of acidic pH and resonance structure:... | Download Scientific Diagram
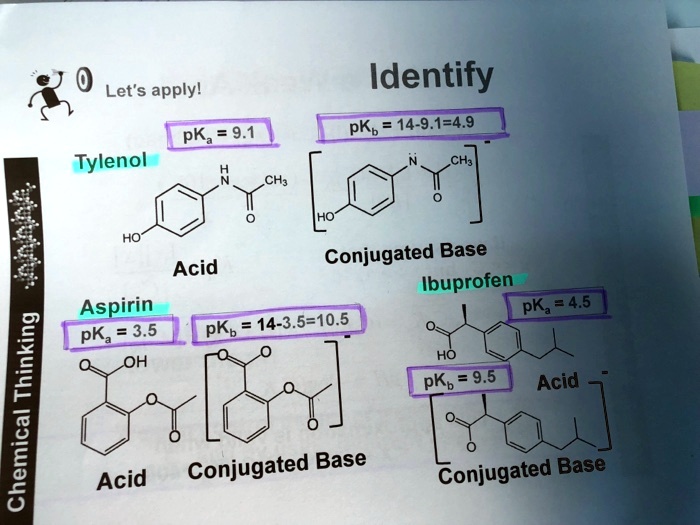
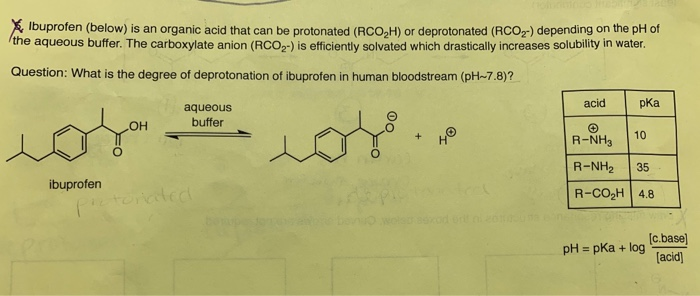
SOLVED: Let's apply. Identify pKb = 14 - 9.1 = 4.9. pKa = 9.1. Tylenol. CH3. CH3. HO. Conjugated Base Acid Ibuprofen Aspirin pKa = 4.5 pKb = 14 - 3.5 - 10.5 pKa OH HO pKb = 9.5 Acid. 1 7 Acid Conjugated Base Conjugated Base.
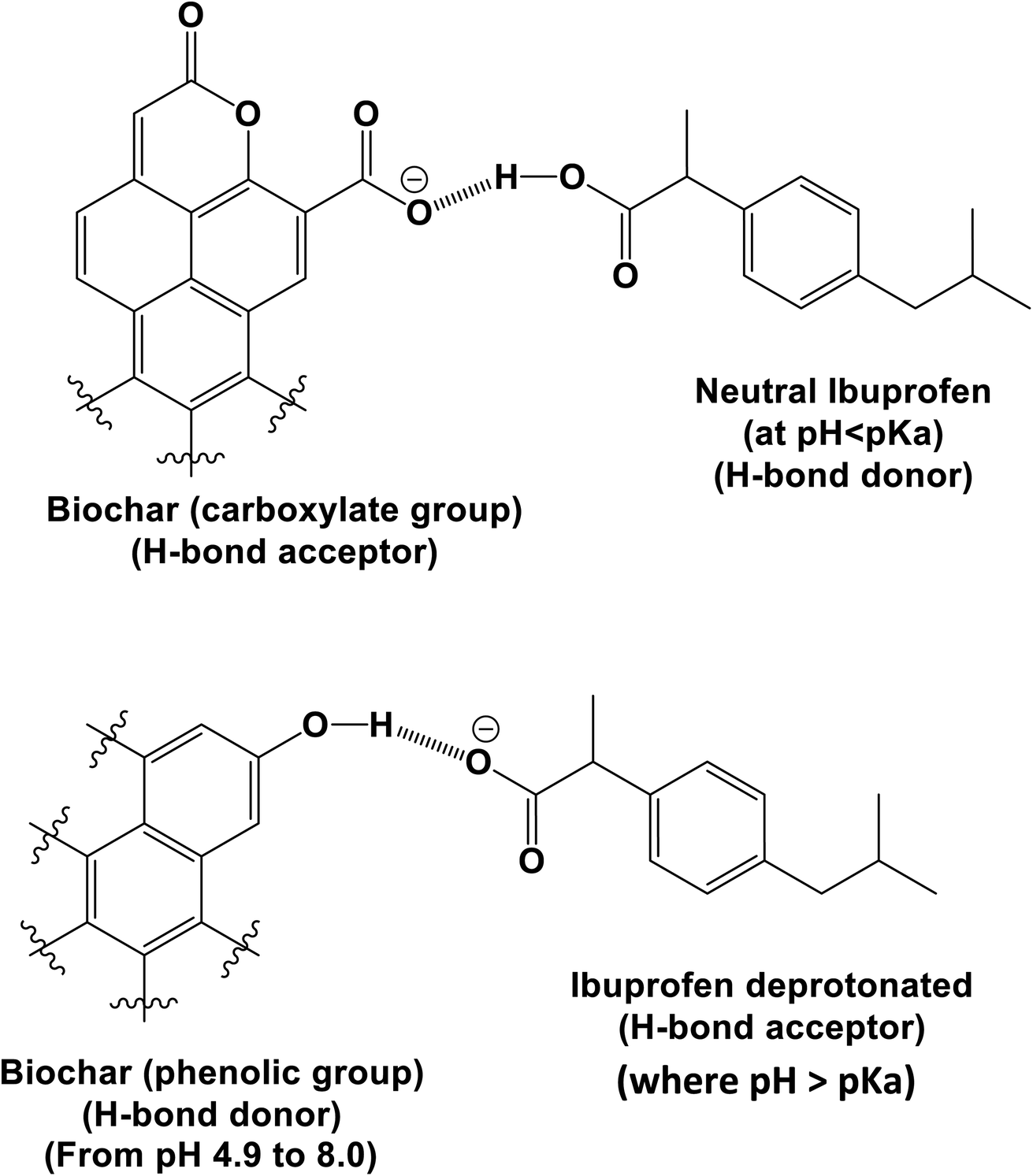
Aqueous ibuprofen sorption by using activated walnut shell biochar: process optimization and cost estimation - Environmental Science: Advances (RSC Publishing) DOI:10.1039/D2VA00015F
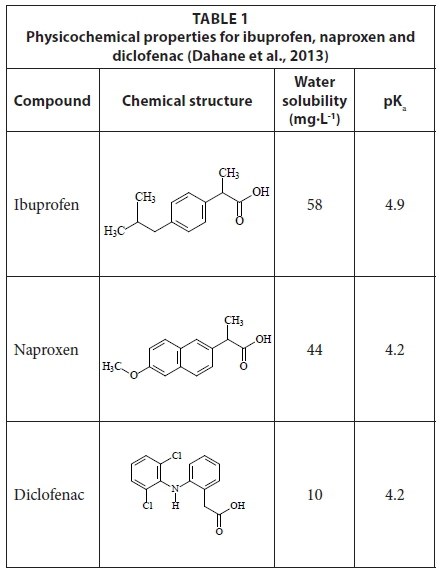
Simultaneous determination of naproxen, ibuprofen and diclofenac in wastewater using solid-phase extraction with high performance liquid chromatography
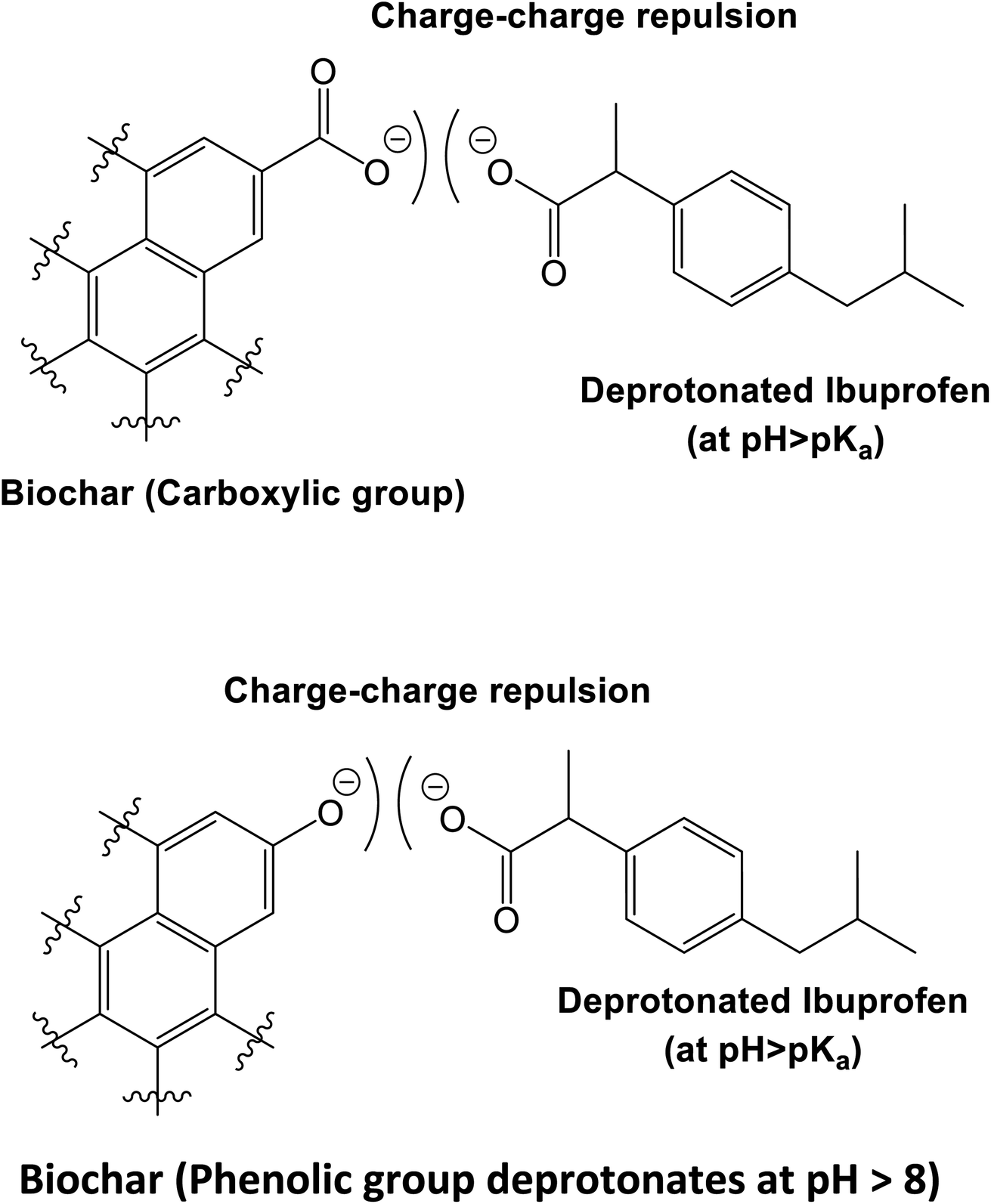
Aqueous ibuprofen sorption by using activated walnut shell biochar: process optimization and cost estimation - Environmental Science: Advances (RSC Publishing) DOI:10.1039/D2VA00015F
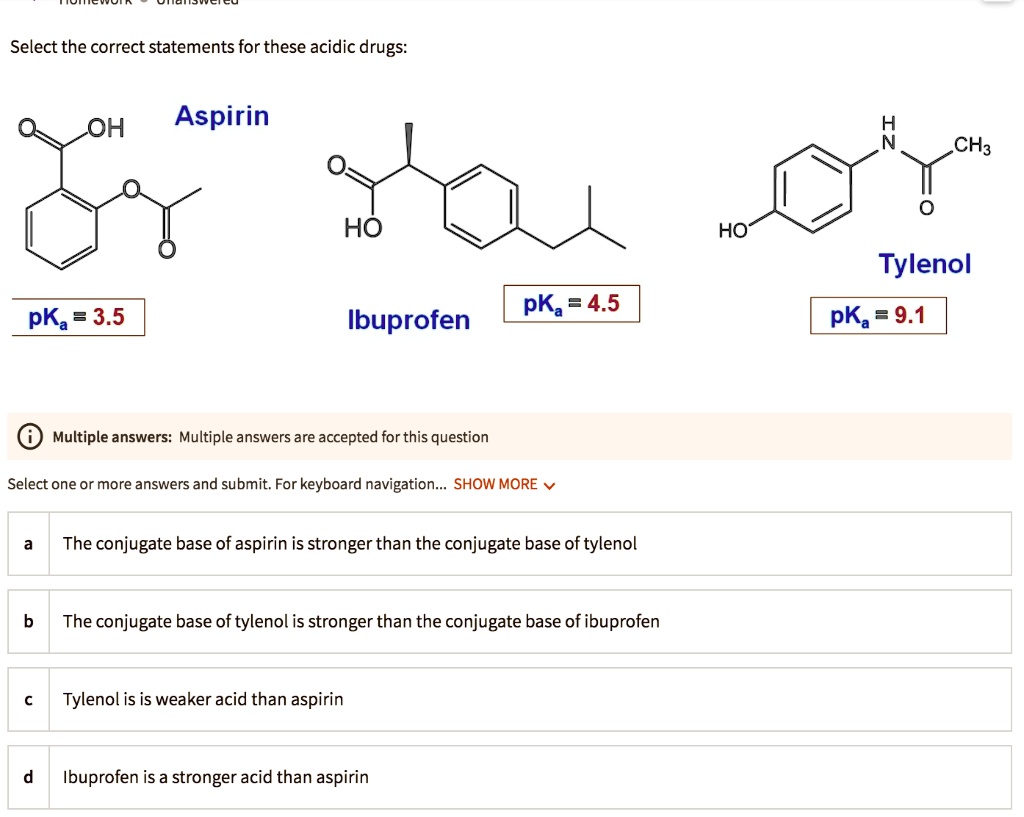
SOLVED: Select the correct statements for these acidic drugs: OH Aspirin HO HO Tylenol pKa 9.1 pKa =4.5 pKa 3.5 Ibuprofen Multiple answers: Multiple answers are accepted for this question Select one
![PDF] Sorption, photodegradation, and chemical transformation of naproxen and ibuprofen in soils and water. | Semantic Scholar PDF] Sorption, photodegradation, and chemical transformation of naproxen and ibuprofen in soils and water. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/bd825006414bdf48d3e25685d998bf571ff563c7/2-Figure1-1.png)